Mechanism Deciphered: How Organic Acids Are Formed in the Atmosphere
An international team of scientists revealed the dominant mechanism in the formation of formic acid which affects cloud formation
The acidity of the atmosphere is increasingly determined by carbon dioxide and organic acids such as formic acid. The second of these contribute to the formation of aerosol particles as a precursor of raindrops and therefore impact the growth of clouds and pH of rainwater. In previous atmospheric chemistry models of acid formation, formic acid tended to play a small role. The chemical processes behind its formation were not well understood. An international team of researchers under the aegis of Forschungszentrum Jülich, including researchers from the Max Planck Institute of Chemistry, has now succeeded in filling this gap and deciphering the dominant mechanism in the formation of formic acid. This makes it possible to further refine atmosphere and climate models. The results of the study have now been published in the journal Nature.

In Germany, we are familiar with acid rain, particularly from our experience in the 1980s. The culprits were nitrogen and sulfur oxides released into the atmosphere by humans which reacted with water droplets in clouds to form nitric and sulfuric acid. Acid rain has a pH (measure of acidity) of about 4.2–4.8, lower than that of pure rainwater (5.5–5.7), which results from the natural carbon dioxide content of the atmosphere. Also, organic acids, and especially formic acid, play an important role in cloud acidification.
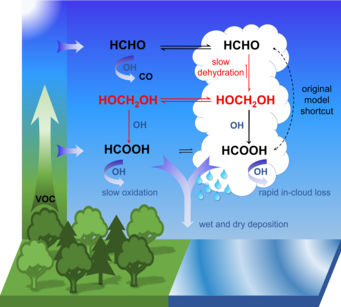
However, the chemical process that forms the bulk of the formic acid present in the atmosphere was unknown up to now. Dr. Bruno Franco and Dr. Domenico Taraborrelli from Jülich’s Institute of Energy and Climate Research – Troposphere have now deciphered it: First, formaldehyde is being formed naturally by photo-oxidation of volatile organic compounds. Second, it reacts in cloud droplets with water molecules to form methanediol. The majority of the latter is out-gassed and reacts with OH radicals, sometimes called the “detergent of the atmosphere”, in a photochemical process to form formic acid. A smaller portion also reacts within the liquid phase in water droplets to form formic acid that is spread by rain.
“According to our calculations, the oxidation of methanediol in the gas phase produces up to four times as much formic acid as what is produced in other known chemical processes in the atmosphere,” says Domenico Taraborrelli. This amount reduces the pH of clouds and rainwater by up to 0.3, which highlights the contribution of organic carbon to the natural acidity in the atmosphere.
Theory check using EMAC

As a first step, the two scientists tested their theory using the global ECHAM5/MESSy Atmospheric Chemistry Model (EMAC), and compared the results with remote sensing data. Dr. Andrea Pozzer, group leader at the MPI for Chemistry, explains: “With the development and constant improvement of EMAC in our institute, we laid the foundations for building the first atmospheric global model capable of explicitly simulating multiphase chemical kinetics.” The EMAC infrastructure (i.e. MESSy) provides the possibility to integrate a large number of sub-models describing the physical and chemical processes occurring in the atmosphere in one model framework and to investigate in detail interactions between the individual processes. “The modelling group of our institute not only contributed to the implementation of fundamental processes for this study, but also to the analysis of the model results which proved the importance of heterogeneous in-cloud formation of formic acid”, he adds.
Subsequent experiments in Jülich’s SAPHIR atmosphere simulation chamber confirmed these results.
“We assume that the mechanism demonstrated is also active in aqueous aerosols and applies to other organic acids such as oxalic acid, which are not adequately accounted for in atmospheric chemistry models to date,” says Taraborrelli. One of the effects of this could be an improved understanding of the growth of aerosol particles and the development of clouds.